JOIN A CONTRACEPTION CLINICAL STUDY AND EARN UP TO $650
Sign Up Today – Space Is Very Limited!
Now you have an opportunity to explore improved treatment options and also earn extra money participating in an FDA-licensed and approved clinical research study.
It’s easy…it’s convenient…and it’s open to you right now.
HERE IS HOW TO GET STARTED
As a study participant, you play a valuable role in helping to get safe, effective drugs approved for public use.
Because you are such an important part of our studies, we have simplified the steps for participation.

Call 1-844-466-4673 or Click on Contraception Study Below
This is the first step. All volunteers will be able to review the Informed Consent form, and the program will be carefully explained by our professional medical team.
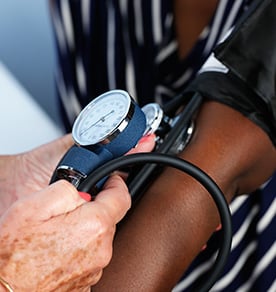
Come in for a FREE Medical Exam
After evaluating your particular medical situation, we will invite you into our clinic for a comprehensive and complimentary physical exam.

No Special Requirements
If you meet the physical guidelines of the study, you will be asked to participate. No insurance, social security number or proof of citizenship is required.

Receive Up to $650
Participants will be assigned to different groups, where you will be carefully monitored for treatment and safety. As a volunteer, you will earn as much as $650 for completing the study.
WHAT YOU SHOULD KNOW
This professional clinical study, conducted by Hope Clinical Research, is a short term trial in which you will intake a promising new medication seeking FDA approval. Depending on the study you join, this medication’s use is intended to effectively treat your medical condition.
The contraception study is a phase 2 study which means that the Phase 1 study was very promising and the FDA has approved the study to move to phase 2. Phase 1 study included human participants and saw limited adverse reaction issues.
Here is what you can expect regarding these studies:
- Complimentary consultation and health exam
- Complimentary comprehensive lab reports
- Complimentary study-related medicines
- Meets all industry safety guidelines
- Governed & monitored by central ethics committee
- Free transportation or travel reimbursement
- FDA-approved
- HIPAA-compliant
- Full confidentiality
- Guaranteed compensation
Hope Clinical Research
A RESPECTED MEDICAL TEAM
Hope Clinical Research has extensive experience conducting Phase II through IV clinical trials with many leading pharmaceutical companies. The facilityls lead investigator, Dr. Hessam Aazami, features many years in clinical research and counts over 8,000 patients in his current practice. Every member of the team offers a wealth of experience, a caring personality and a commitment to ably serving the pharmaceutical industry, the community and its patients.

Dr. Aazami brings 8 years of research experience working at UCLA and USC. He speaks English, Spanish, Italian and Farsi.
Hope Clinical Research
22030 Sherman Way Suite 101
Canoga Park, CA 91303
Call Toll-Free: 844-466-4673
Email: [email protected]
Reserve Your Place Now…
Complete the short form below

